
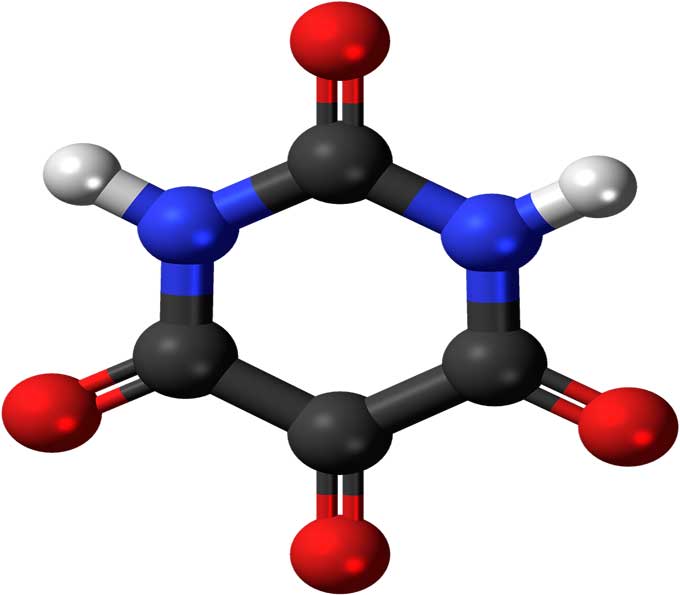
It requires both appreciation of many critical details and some sophisticated reasoning, making it complicated and hard for a large majority of the general student population. Finally, the pros and cons of a modern qualitative quantum mechanical approach to bonding are considered.Īlthough the concept of chemical bonding is fundamental to the teaching of chemistry at both the secondary and the tertiary levels, the topic has proved difficult to organize and approach for curriculum designers, teachers, and students alike. A learning progressions approach, employing lower and upper anchors of relevant scientific knowledge is considered, and a proposed list of potential core concepts, lever concepts, and stepping-stones are presented. We recommend that a spiral curriculum spanning all three upper-secondary grades should be adopted. In the present study, we review earlier studies, which made proposals concerning the teaching of this topic, and provide some proposals of our own, based on the findings of our previous study. In addition, we presented an enriched teaching text on this topic for the tenth grade and examined its effectiveness with regard to the same aspects of bonding. Chemistry Education Research and Practice, 19(4), 1253–1269), we reviewed previous studies on students’ misconceptions and conceptual difficulties with the topic of chemical bonding and tested the knowledge of tenth-grade Greek students on certain key aspects of bonding.
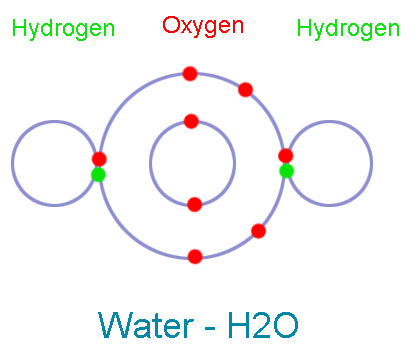

Teaching and learning chemical bonding: Research-based evidence for misconceptions and conceptual difficulties experienced by students in upper secondary schools and the effect of an enriched text. In a preceding publication (Tsaparlis, G., Pappa, E.


 0 kommentar(er)
0 kommentar(er)
